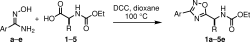
42.
Copper-Mediated Intramolecular Interrupted CuAAC Selanylation
Wystan K. O. Teixeira, W. K. O.; de Albuquerque, D. Y.; Zukerman-Schpector, J.; Seckler, D.; Rampon, D. S.; Schwab, R. S.*
J. Org. Chem. 2023, 88, 10434–10447

41.
Métodos Sintéticos Verdes: Algumas Contribuições do CERSUSCHEM
Correa, A. G.; Paixão, M. W.; Schwab, R. S.
In: Química Orgânica Sintética, Brasil; Rosa, F. A.; da Silva, F. de C.; Amarante, G. W.; de Oliveira, K. T.; Victor, M. M.; Cunha, S. do D. E-Papers, 2022, 205-232.

40.
Recent Developments on Palladium-Catalyzed Carbonylation Reactions in Renewable Solvents
de Albuquerque, D. Y.; Teixeira, W. K. O.; Narayanaperumal, S.; Schwab, R. S.*
J. Braz. Chem. Soc. 2022, 33, 637-663

38.
The crystal structure of 1-(2-iodophenyl)- 4-phenyl-1H-1,2,3-triazole, C14H10IN3
Caracelli, I.* Teixeira, W. K. O.; de Albuquerque, D. Y.; Schwab, R. S.; Tiekink, E. R. T.
Z. Kristallogr. - N. Cryst. Struct. 2022; 237, 1093-1096
37.
Palladium-Catalyzed Carbonylative Synthesis of Aryl Selenoesters Using Formic Acid as an Ex Situ CO Source
de Albuquerque, D. Y.; Teixeira, W. K. O.; do Sacramento, M.; Alves, D.; Santi, C.; Schwab, R. S.*
J. Org. Chem. 2022, 87, 595-605

36.
One-pot synthesis of 1,2,4-oxadiazoles from chalcogen amino acid derivatives under microwave irradiation
Wolf, L.; Mayer, J. C. P.; Quoos, N.; Sauer, A. C.; Schwab, R. S.; Rodrigues, O. E. D.; Dornelles, L.*
Tetrahedron, 2021, 91, 132222

35.
Development of a Novel Three-step Sonogashira Cross Coupling/Deacetonation/Cycloaddition Protocol for the Synthesis of 4-aryl-1,2,3-triazoles Using 2-methyl-3-butyn-2-ol as a Versatile Acetylene Surrogate
da Silva, E. M.; de Albuquerque, D. Y.; Zukerman-Schpector, J.; Schwab, R. S.*
Asian J. Org. Chem. 2021, 10, 1153-1160

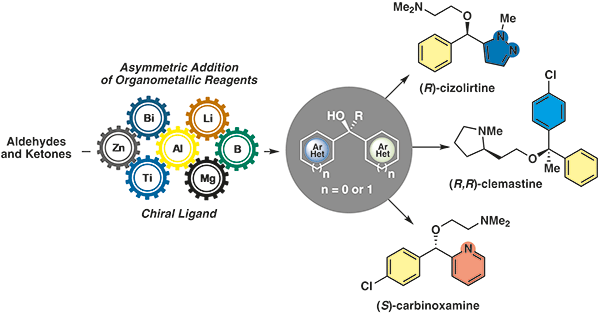
34.
Recent Advances in the Synthesis of Enantiomerically Enriched Diaryl, Aryl Heteroaryl, and Diheteroaryl Alcohols through Addition of Organometallic Reagents to Carbonyl Compounds
Teixeira, W. K. O.; de Albuquerque, D. Y.; Narayanaperumal, S.; Schwab, R. S.*
Synthesis 2020, 52, 1855-1873
33.
Catálise heterogênea: aplicação de zeólitas multifuncionais na valorização de biomassa
Teixeira, W. K. O.; de Albuquerque, Schwab, R. S.*
In: Biomassa: estrutura, propriedades e aplicações.; Correa, A. G.; Gallo, J. M. R.; EdUFSCar, 2020, 205-232.
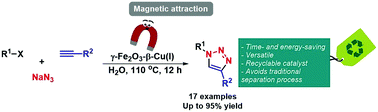
32.
Greener synthesis of 1,2,3-triazoles using a copper(I)-exchanged magnetically recoverable b-zeolite as catalyst
Costa, E. R.; Andrade, F. C. D.; de Albuquerque, D. Y.; Ferreira, L. E. M.; Lima, T. M.;* Lima, C. G. S.; Silva, D. S. A.; Urquieta-González, E. A.; Paixão, M. W.; Schwab, R. S.*
New J. Chem. 2020, 44, 15046-15053
31.
2-Methyl-4-(4-nitrophenyl)but-3-yn-2-ol: crystal structure, Hirshfeld surface analysis and computational chemistry study
Caracelli, I.; Zukerman-Schpector, J.; Schwab, R. S.; da Silva, E. M.; Jotanic, M. M.; Tiekink, E. R. T. *
Acta Cryst. 2019, E75, 1232–1238
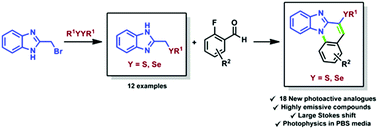
30.
Straightforward synthesis of photoactive chalcogen functionalized benzimidazo[1,2-a]- quinolines
da Silva, R. B.; Coelho, F. L.; Rodembusch, F. S.; Schwab, R. S.; Schneider, J. M. F. M.; Rampon, D. da S.; Schneider, P. H.*
New J. Chem., 2019, 43, 11596-11603
29.
Palladium-Catalyzed Aminocarbonylation Reaction to Access 1,2,3-Triazole-5-carboxamides Using Dimethyl Carbonate as Sustainable Solvent
de Albuquerque, D. Y.; de Moraes, J. R.; Schwab, R. S.*
Eur. J. Org. Chem. 2019, 6673-6681

28.
A Straightforward Sequential Approach for the Enantioselective Synthesis of Optically Active α-Arylmethanol-1,2,3-Triazoles
Andrade, F. C. D.; Pugnal, L. V. B. L.; Betim, H. L. I.; Vani, J. F.; Zukerman-Schpector, J.; Schwab, R. S.*
Eur. J. Org. Chem. 2018, 5467-5476


27.
4-(4-Acetyl-5-methyl-1H-1,2,3-triazol-1-yl)benzonitrile: crystal structure and Hirshfeld surface analysis
Zukerman-Schpector, J.;* Dias, C. da S.; Schwab, R. S.; Jotanic, M. M.; Tiekink, E. R. T.
Acta Cryst. E74, 2018, 1195-1200
26.
CuO Nanoparticles as An Efficient Heterogeneous Catalyst for the 1,3-Dipolar Cycloaddition of Dicarbonyl Compounds to Azides
Dias, C. da. S.; Lima, T. de M.; Lima, C. G. S.; Zukerman-Schpector, J.; Schwab, R. S.*
ChemistrySelect 2018, 3, 6195-6202

25.
Factorial design evaluation of the Suzuki cross-coupling reaction using a magnetically recoverable palladium catalyst
Guerra, R. R. G, Martins, F. C. P.; Lima, C. G. S.; Gonçalves, R. H.; Leite, E. R.; Pereira-Filho, E. R.; Schwab, R. S.*
Tetrahedron Lett. 2017, 58, 903-908


24.
Straightforward synthesis and antioxidant studies of chalcogenoaziridines
Borges, R.; Andrade, F. C. D.; Schwab, R. S.;* Sousa, F. S. S.; de Souza, M. N.; Savegnago, L.; Schneider, P. H.*
Tetrahedron Lett. 2016, 57, 3501-3504

23.
Synthesis of Biologically Active Selenium-Containing Molecules From Greener Perspectives
Azeredo, J. B.; Schwab, R. S.; Braga, A. L.*
Current Green Chemistry, 2016, 3, 51-67
22.
Synthesis of 1,3,4-oxadiazole derivatives from -amino acid and acyl hydrazides under thermal heating or microwave irradiation conditions
Dornelles, L.;* Rodrigues, O. E. D.; Heck, E. F.; Bender, C. R.; Cansian, M. B.; Schwab, R. S.; Filho, W. A .S
ARKIVOC 2015 (vii) 131-144

21.
Application of Bio-Based Solvents in Catalysis
Corrêa, A. G.;* Paixão, M. W.;* Schwab, R. S.*
Current Organic Synthesis, 2015, 12, 675-695

20.
Effect of diselenide administration in thioacetamide-induced acute neurological and hepatic failure in mice
Stefanello, S. T.; da Rosa, E. J. F.; Dobrachinski, F.; Amaral, G. P.; de Carvalho, N. R.; da Luz, S. C. A.; Bender, C. R.; Schwab, R. S.; Dornelles, L.; Soares, F. A. A.*
Toxicol. Res., 2015, 4, 707-717
19.
Highly Efficient and Magnetically Recoverable Niobium Nanocatalyst for the Multicomponent Biginelli Reaction
Lima, C. G. S.; Silva, S.; Gonçalves, R. H.; Leite, E. R.; Schwab, R. S.; Corrêa, A. G.; Paixão, M. W.*
ChemCatChem 2014, 6, 3455-3463

18.
Eco-Friendly Access and Application of Organoselenium Reagents: Advances Toward Green Chemistry
Braga, A. L.; Schwab, R. S.; Rodrigues, O. E. D.
In: Organoselenium Chemistry: Between Synthesis and Biochemistry. 1ed.: Bentham Science, 2014, v. , p. 197-267.

17.
Anxiolytic effects of diphenyl diselenide on adult zebrafish in a novelty paradigm
Ibrahim, M.; Mussulini, B. H. M.;* Moro, L.; de Assis, A. M.; Rosemberg, D. B.; de Oliveira, D. L.; Rocha, J. B. T.; Schwab, R. S.; Schneider, P. H.; Souza, D. O.; Rico, E. P.
Progress in Neuro-Psychopharmacology & Biological Psychiatry, 2014, 54, 187-194
16.
Straightforward synthesis of non-natural L-chalcogen and L-diselenide N-Boc-protected-γ-amino acid derivatives
Kawasoko, C. Y.; Foletto, P.; Rodrigues, O. E. D.; Dornelles, L.; Schwab, R. S.;* Braga, A. L.*
Org. Biomol. Chem., 2013, 11, 5173-5183

15.
Synthesis of Thiol Esters Using Nano CuO/Ionic Liquid as an Eco-Friendly Reductive System Under Microwave Irradiation
Azeredo, J. B.; Godoi, M.; Schwab, R. S.; Botteselle, G. V.; Braga, A. L.*
Eur. J. Org. Chem. 2013, 5188-5194
.jpg)
14.
Evaluation of in vitro antioxidant effect of new mono and diselenides
Stefanello, S. T.; Prestes, A. S.; Ogunmoyole, T.; Salman, S. M.; Schwab, R. S.; Brender, C. R.; Dornelles, L.; Rocha, J. B. T.; Soares, F. A. A.*
Toxicology in VitroI, 2013, 27, 1433-1439
13.
Straightforward synthesis of non-natural chalcogen peptides via ring opening of aziridines
Schwab, R. S.;* Schneider, P. H.*
Tetrahedron, 2012, 68, 10449-10455

12.
Antioxidant activity of b-selenoamines and their capacity to mimic different enzymes
Prestes, A. de S.; Stefanello, S. T.; Salman, S. M.; Pazini, A. M.; Schwab, R. S.; Braga, A. L.; Barbosa, N. B. de V.; Rocha, J. B. T.
RSC Adv., 2012, 2, 8478–8482
11.
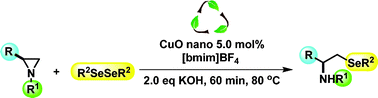
CuO nano particles and [bmim]BF4: an application towards the synthesis of chiral b-seleno amino derivatives via ring opening reaction of aziridines with diorganyl diselenides
Salman, S. M.; Narayanaperumal, S.; Schwab, R. S.;* Bender, C. R.; Rodrigues, O. E. D.;* Dornelles, L.*
RSC Adv., 2012, 2, 8478–8482
10.
Ephedrine-based diselenide: a promiscuous catalyst suitable to mimic the enzyme glutathione peroxidase (GPx) and to promote enantioselective C–C coupling reactions
Soares, L. C.; Alberto, E. E.; Schwab, R. S.; Taube, P. S.; Nascimento, V.; Rodrigues O. E. D.; Braga, A. L.*
Org. Biomol. Chem., 2012, 10, 6595–6599
9.
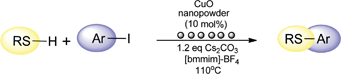
C–S cross-coupling of thiols with aryl iodides under ligand-free conditions using nano copper oxide as a recyclable catalyst in ionic liquid
Schwab, R. S.; Singh, D.; Alberto, E. E.; Piquini, P.; Rodrigues, O. E. D.;* Braga, A. L.*
Catal. Sci. Technol., 2011, 1, 569–573
8.
Efficient Ring Opening of Protected and Unprotected Aziridines Promoted by Stable Zinc Selenolate in Ionic Liquid
Salman, S. M.; Schwab, R. S.; Alberto, E. E.; Vargas J.; Dornelles, L.;* Rodrigues, O. E. D.;* Braga, A. L.*
Synlett, 2011, 50, 0069–0072

7.
Chiral Chalcogen Peptides as Ligands for the Catalytic Enantioselective Aryl Transfer Reaction to Aldehydes
Schwab, R. S.; Soares, L. C.; Dornelles, L.; Rodrigues, O. E. ;* Paixão, M. W.; Godoi, M.; Braga, A. L.*
Eur. J. Org. Chem. 2010, 3574–3578
.jpg)
6.
Synthesis of selenium- and tellurium-containing nucleosides derived from uridine
Braga, A. L.;* Filho, W. A. S.; Schwab, R. S.; Rodrigues, O. E. D.; Dornelles, L.; Braga, H. C.; Lüdtke, D. S.*
Tetrahedron Letters, 2009, 50, 3005–3007

5.
Genotoxicity of organoselenium compounds in human leukocytes in vitro
Santos, D. B.; Schiar, V. P. P.; Ribeiro, M. C. P.; Schwab, R. S.; Meinerz, D. F.; Allebrandt, J.; Rocha, J. B. T.; Nogueira, C. W.; Aschner, M.; Barbosa, N. V. B.∗
Mutation Research 2009, 676, 21-26
4.
Ring opening of unprotected aziridines by zinc selenolates in a biphasic system
Braga, A. L.;* Schwab, R. S.; Alberto, E. A.; Salman, S. M.; Vargas, J.; Azeredo, J. B.
Tetrahedron Letters, 2009, 50, 2309–2311

3.
A convenient synthetic route for alkynylselenides from alkynyl bromides and diaryl diselenides employing copper(I)/imidazole as novel catalyst system
Sharma, A.;* Schwab, R. S.; Braga, A. L.;* Barcellos, T. Paixão, M. W.
Tetrahedron Letters, 2008, 49, 5172–5174

2.
Organocatalytic asymmetric aldol reactions mediated by a cysteine-derived prolinamide
Schwab, R. S. Galetto, F. Z.; Azeredo, J. B.; Braga, A. L.; Lüdtke, D. S.;* Paixão, M. W.*
Tetrahedron Letters, 2008, 49, 5094–5097

1.
‘One-Pot’ Synthesis of Chiral N-Protected a-Amino Acid-Derived 1,2,4-Oxadiazoles
Braga, A. L.;* Lüdtke, D. S.; Alberto, E. E.; Dornelles, L.;* Filho, W. A. S.; Corbellini, V. A.; Rosa, M.; Schwab, R. S.
Synthesis, 2004, 1589-1594